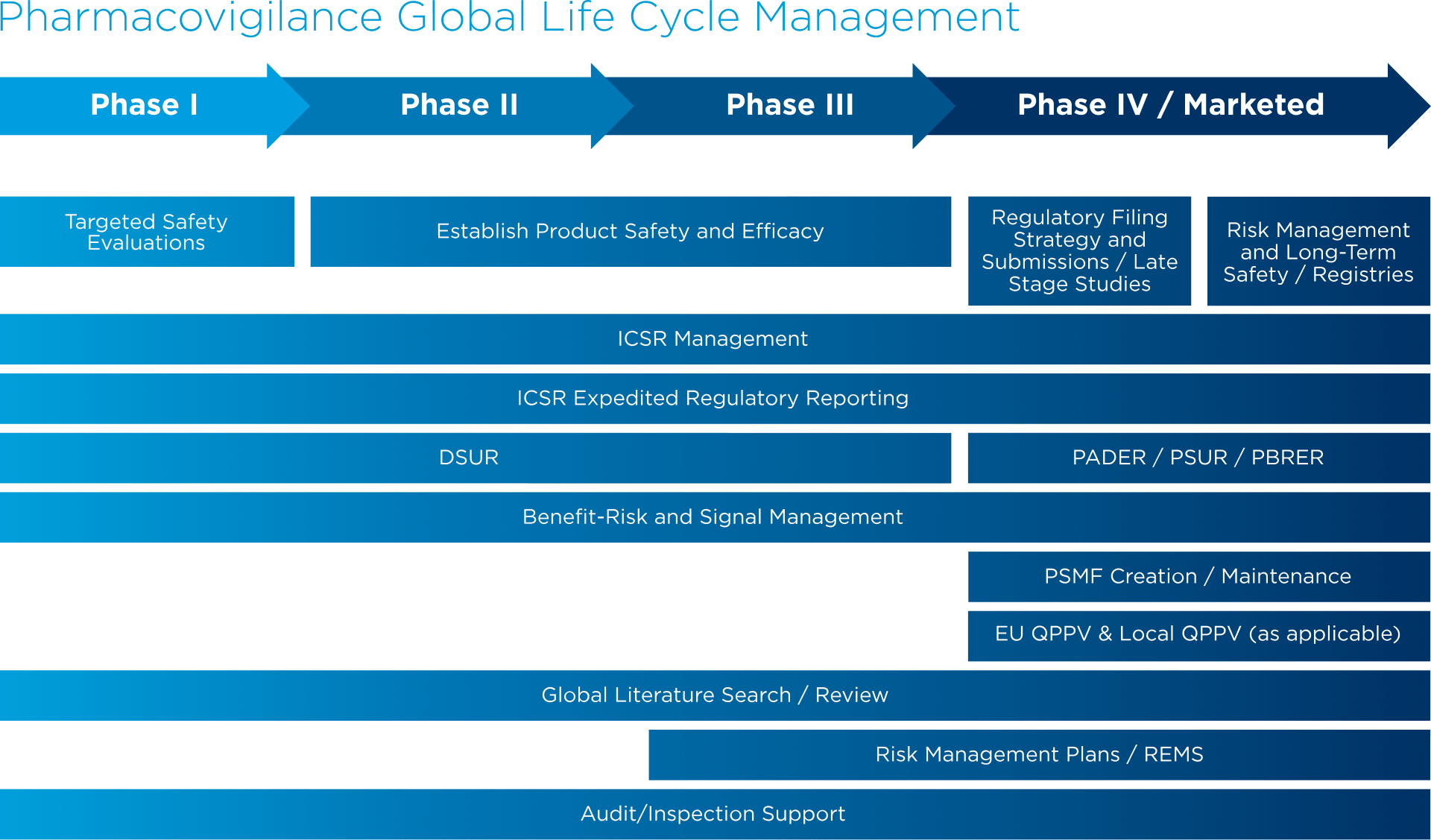
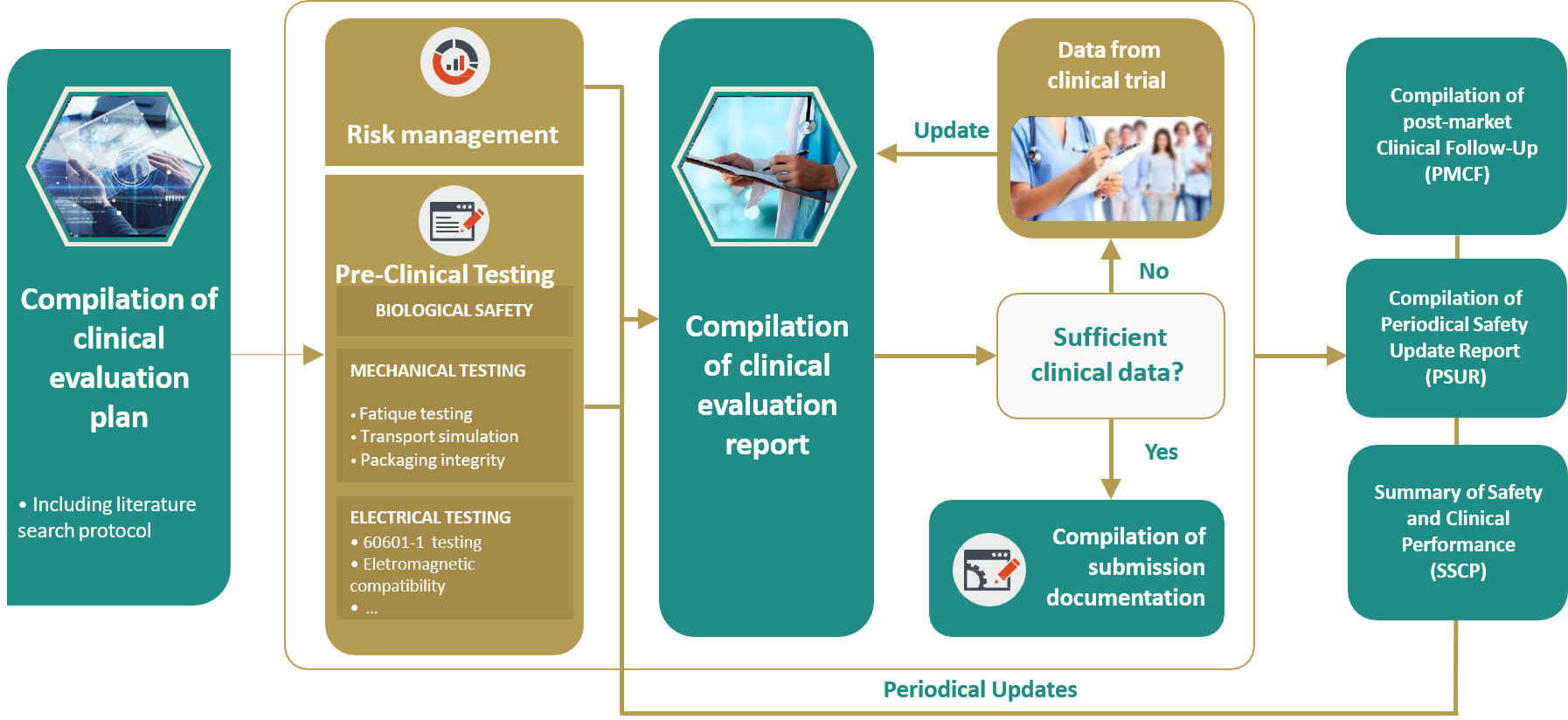
Global Healthcare Brand Improves Safety Reporting in Clinical Trials Leveraging Pharmacovigilance Analytics| Quantzig | Business Wire

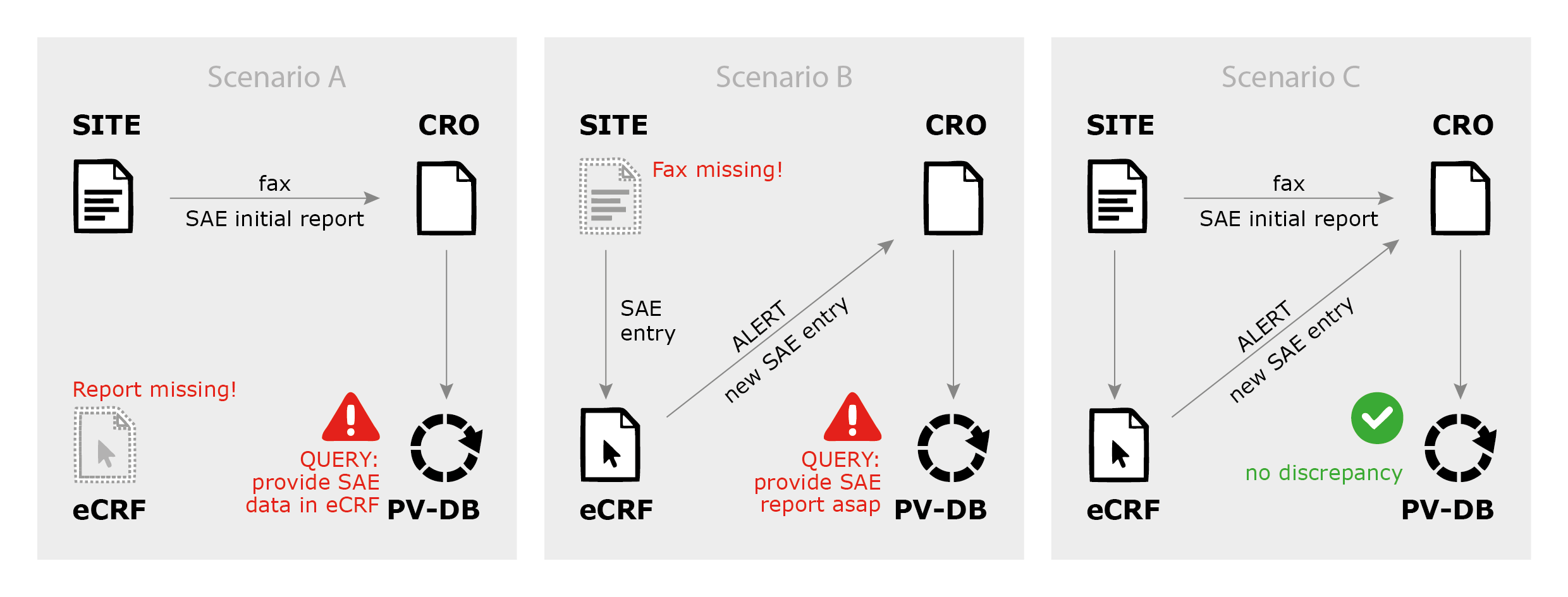
Simplifying safety reporting as part of clinical trial investigator retention - Drug Discovery and Development